What is Dementia, and what’s the difference between Alzheimer’s and Dementia?
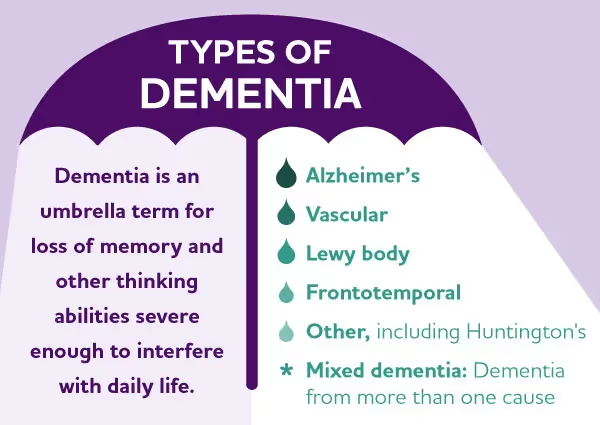
Dementia is an umbrella term used to describe a group of symptoms related to a progressive decline in cognitive abilities, including memory, language, problem-solving, behavior, and function that impact activities of daily living. According to the Alzheimer’s Association, about 6.7 million seniors aged 65+ have Alzheimer’s disease and related dementias. The most common types of dementia include Alzheimer’s disease, vascular dementia, Lewy body, and Frontotemporal dementia. Alzheimer’s disease accounts for about 60-80% of dementia cases. Vascular dementia, which occurs because of microscopic bleeding and blood vessel blockage in the brain, is the second most common cause of dementia.
Progression
Alzheimer’s disease progresses in stages with a range of symptoms that increase in severity over time. Because the disease affects people in different ways, the rate of progression will vary. On average, a person with Alzheimer’s may live four to eight years after diagnosis, but some people live as long as 20 years. Stages of Alzheimer’s may overlap, which can make it difficult to know which stage a person is in.
- Asymptomatic: On the earliest end of the continuum are people who are asymptomatic (i.e., without symptoms). This means that they may have the biological changes of the disease (for example, the buildup of beta-amyloid protein in the brain) in their brain but do not show any cognitive symptoms. There are clinical trials enrolling volunteers without any changes in memory or thinking to assess whether they may already have elevated brain amyloid, and evaluating potential treatments to prevent Alzheimer’s-related symptoms from developing.
- Mild cognitive impairment (MCI) due to Alzheimer’s: Mild cognitive impairment (MCI) is an early stage of memory loss or other loss of cognitive ability in individuals who can still independently perform activities of daily living. MCI can develop for multiple reasons, and some individuals living with MCI may go on to develop dementia while others will not. MCI can be an early stage of Alzheimer’s disease if hallmark changes in the brain, such as beta-amyloid buildup, are present. Many patients in this stage are not diagnosed, as their changes in memory and thinking are not severe enough to interfere with many daily activities. There are many Alzheimer’s clinical trials enrolling patients in this stage of the disease, as it may be a critical point at which treatments could prevent or delay progression to dementia.
- Mild dementia due to Alzheimer’s disease (early): If hallmark changes in the brain are present, the person may progress into dementia due to Alzheimer’s disease. A person with mild dementia due to Alzheimer’s (sometimes referred to as the early stage) will typically start to experience symptoms that interfere with some daily activities.
- Moderate dementia due to Alzheimer’s disease (middle): For those with moderate dementia due to Alzheimer’s disease (sometimes referred to as the middle stage), biological changes in the brain continue to progress, and symptoms are more pronounced and will interfere with many of the person’s daily activities. This is typically the longest stage of the disease and can last for many years.
- Severe dementia due to Alzheimer’s disease (late): In this stage (sometimes referred to as the late stage), biological changes in the brain continue to progress. Symptoms are severe and will interfere with most daily activities. People in this stage lose the ability to carry on a conversation, respond to the environment, and, eventually, control movement. Assistance or supervision is required to complete most daily personal care.
Why are Clinical Trials Important?
According to the National Institutes of Health (NIH): “Clinical trials are… at the heart of all medical advances.” Clinical trials look at new ways to prevent, detect, or treat disease and they assess if a therapy is safe and if it works (efficacy).
To evaluate if a drug works, the US Food & Drug Administration (FDA) assesses if an investigational therapy has a “clinically meaningful benefit.” In essence, can it improve your quality of life, or have a positive effect on how you “feel, function, or survive".
Clinical trials help researchers find better treatments for people in the future, but the NIH also acknowledges they “offer hope for many people” fighting diseases like Alzheimer’s disease and related dementias today.
That’s why our team at Synapticure prioritizes discussing clinical trial options with you, helping you optimize your chances of qualifying for the best trial for you.
Common Criteria for Enrollment in a Clinical Trial
Each trial has specific inclusion and exclusion criteria based on factors like age, stage of Alzheimer’s, overall health, and previous treatments.
Common Inclusion Criteria:
- Diagnosis or Risk Factors: Participants often need a confirmed diagnosis of Alzheimer's or a specific stage (e.g., mild cognitive impairment, early Alzheimer’s disease, or moderate Alzheimer's disease). Diagnosis may involve cognitive assessments, brain imaging (e.g., MRI, PET scans), or biomarker testing (e.g., amyloid-beta or tau levels). Often this confirmation occurs during the screening stage of study participation, so you don’t need a previous diagnosis or testing to qualify for study screening.
- A Study Partner: Most studies related to dementia require that a study participant have a “study partner” who can answer some questions about the participant’s usual activities and any changes that occur over time. Often studies will require that a study partner is someone who has at least 10 hours of contact with the participant per week and can be a spouse, relative, adult child, or close friend.
- Age: Most trials include participants over a certain age, typically 50 to 85 years, as Alzheimer’s primarily affects older adults.
- Stable Medical Condition: Participants should be in stable general health, with any chronic medical conditions (like hypertension or diabetes) being well-managed.
- Medications: Most studies will require that participants be on stable doses of their current medications for a certain period of time before entering the study (often at least 3 months). The use of specific medications may be restricted, especially medications that can affect memory and thinking, like antipsychotics and sedatives.
Common Exclusion Criteria
- Other Forms of Dementia: Participants with other types of dementia (e.g., vascular dementia, Lewy body dementia, or frontotemporal dementia) are often excluded to ensure the study focuses specifically on Alzheimer's disease.
- Severe or Unstable Medical Conditions: Those with serious, unstable medical conditions (e.g., heart failure, kidney disease, or uncontrolled diabetes) may be excluded due to the increased risk of complications during the study. Recent major surgeries or hospitalizations may also be grounds for exclusion.
- Other Neurological or Psychiatric Disorders: Participants with Parkinson’s disease, stroke, major depression, or schizophrenia may be excluded, as these conditions can make it difficult to measure cognitive changes over time.
Understanding Goals of Different Phase Trials
Clinical trials are run in multiple steps, called phases. Each phase has a different purpose and helps researchers answer different questions about an investigational therapy. As a drug progresses through each phase of clinical trials, we learn more about both its safety and efficacy.
To help you understand which trial might be best for you, it’s important to understand the different phases of trials.
- Phase 1 - Phase 1 trials study the investigational drug in humans for the first time. The purpose is to learn about the drug’s safety, to identify side effects, and to determine how the drug is metabolized and excreted. In other diseases, the study population is often 20-100 people. In Alzheimer’s disease research, the size of Phase 1 trials is usually under 100 patients. Phase 1 trials are not “powered for efficacy,” which means there are not enough people participating in the trial to reach any reliable statistical conclusions about whether it works in humans.
- Phase 2 - This phase emphasizes efficacy. The investigational drug is tested in a larger group of people to determine if there is evidence of efficacy, to assess dosing options, and to further study safety. In Alzheimer’s disease research, the size of Phase 2 trials is typically between 100-300 patients.
- Phase 3 - The investigational drug is studied to determine whether there is “substantial evidence” to confirm its efficacy, to continue to monitor side effects, to compare it with standard treatments on the market, and to weigh the drug’s risks versus benefits. In Alzheimer’s, the size of phase 3 trials is typically between 300-2,500 patients.
Depending on your preferences, you may want to join an earlier or later phase. For example, Phase III trials are more likely to offer treatments that are closer to becoming widely available.
How to find a trial:
ClinicalTrials.gov: This government website provides a comprehensive list of ongoing and upcoming trials, searchable by location, disease, and eligibility criteria.
Alzheimer's Association TrialMatch®: This free service matches patients with clinical trials based on the individual's specific diagnosis and health status.
How to choose the right trial for you
- Consult with a Healthcare Provider: Start with talking to a specialist in Alzheimer's care. Clinical trials often have strict inclusion and exclusion criteria, and your care team can help determine if participating in a trial is appropriate based on your current condition, stage of Alzheimer’s, and overall health. If you see a Synapticure provider, we discuss clinical trial options for each patient at their regular visits, so that patients always know what research options are available to them.
- Understand the Trial’s Design and Purpose:
- Purpose: What does the trial aim to achieve? Is it testing a new treatment, a preventive strategy, or a diagnostic tool? Is the trial focused on slowing disease progression, improving existing symptoms, or studying a new diagnostic approach?
- Trial Type: is the study testing an intervention (a treatment or medication) or is it observational (gathering information without adding a treatment)?
- Placebo: Randomized controlled trials are the “gold standard” in clinical research, and in these studies, some patients are assigned to receive a placebo (an inactive substance that looks the same as the study medication). You will want to understand the chances of being assigned to receive the study medication/treatment versus the placebo.
- Understand Potential Risks and Benefits: A trial may offer early access to new treatments and expert care. However, it’s important to recognize that not all treatments will be effective, and some might not work for everyone. Investigate possible side effects or the potential emotional toll of being part of an experimental trial. Always weigh these against potential benefits.
- Logistics: Location and Time
- Location: Some studies require patients to enroll at specific clinical sites, which could involve travel and financial considerations if you don’t live near an enrolling site.
- Time: Trials can vary significantly in the length of the study and the frequency of required visits. Make sure you understand the study schedule for enrolled participants and the length of time required for the visits.
Synapticure’s Cognitive Care Program
Synapticure is a virtual clinic that specializes in caring for people with neurodegenerative conditions like Alzheimer’s and related dementias. Our team of expert clinicians and care navigators work closely with your current healthcare team to make sure you and your loved ones get the care you need. Here’s why families choose us:
- Wraparound support: We are with you every step of the journey and ensure you receive the care you need right when you need it. We help you optimize available insurance benefits, access mental health support, schedule in-home PT/OT, and more.
- Dedicated Dementia specialists: Our team of experts, including neurologists, neuropsychologists, psychiatrists, nurses, and others are equipped to address the diverse needs of patients living with dementia and are available within a week of consultation.
- Accessible care: With licensed providers available in all 50 states and enabled by telehealth, we guarantee timely appointments and support, regardless of location so you don’t have to travel miles for your care.
As a company founded by a patient and caregiver, we understand what you’re going through. Whether you’re newly diagnosed or caring for a loved one, we’re here to help.
Guiding an Improved Dementia Experience (GUIDE)
GUIDE is a new Medicare program designed to enhance the quality of life for patients living with dementia and their caregivers—at no extra cost. This initiative provides comprehensive dementia care management, aiming to reduce the burden on unpaid caregivers and help people with dementia remain safely in their homes and communities. Through the GUIDE program, patients and their families gain access to comprehensive care coordination, as well as access to in-home or facility-based respite care.
With licensed providers in all 50 states, Synapticure will enable eligible patients and their families to access the benefits of the GUIDE Program at no extra cost, which include:
- Local Services Coordination: Assistance in scheduling visits, arranging transportation, and coordinating local services.
- Respite Care: Up to 80 hours per year of respite care covered by Medicare, providing temporary relief options for unpaid caregivers to rest and recharge while ensuring continuous care for their loved ones.
- 24/7 Phone Line: Around-the-clock access to a helpline.
- Care Navigation: Help with medication and meal deliveries, in-home safety assessments, and comprehensive caregiver support and education.
- Expert Care: Access to specialized neurologists and mental health specialists within a week of consultation. Receive personalized care plans to support diagnosis and treatment of physical, cognitive, and behavioral symptoms.
To participate in GUIDE, individuals must meet the following criteria:
- Enrolled in Traditional Medicare Part A and B (not Medicare Advantage)
- Suspected or confirmed diagnosis of Dementia
- Residing at home or within the community (not in long-term care)
- Not currently enrolled in Hospice or a PACE organization
For individuals who do not qualify for GUIDE, Synapticure is still able to provide ongoing care for those living with Dementia and their caregivers. We are happy to discuss your care options. To learn more click here or call us at (708) 249-5450.
ABOUT THE AUTHOR
Amanda Fletcher, MD recently joined Synapticure’s Cognitive Neurology team. Prior to joining Synapticure, Dr. Fletcher specialized in treating people with Alzheimer’s and various related dementias, both in general practice and at ADRCs. She is passionate about addressing health disparities in cognitive neurology and is involved working in communities to help increase clinical trial interest and enrollment. Dr. Fletcher received her undergraduate degree from Jackson State University and her medical degree at Meharry Medical College. She completed her Neurology Residency at the prestigious Barrow Neurological Institute and her fellowship training in Behavioral Neurology and Neuropsychiatry at the renowned Cleveland Clinic Lou Ruvo Center for Brain Health.











.png)


