Dr. Martina de Majo and I were honored to recently host Dr. Jens Schwamborn of OrganoTherapeutics who took us on a fascinating journey of how patient-derived cells can be used to discover promising new therapies in Parkinson’s Disease (PD). OrganoTherapeutics uses cutting-edge human-specific brain organoids to screen for novel compounds which can be therapeutic for different patient subgroups. We’ve provided a summary below and more detail can be found in the recording above.
So, what IS an “organoid”?
Thanks to new, exciting technology, it is possible to create stem cells from any person, usually from a skin sample or blood (you can read more about it here). Stem cells are cells that have the ability to become any number of different cell types including different kinds of brain cells. Once these brain cells are grown, they can organize into small (~2mm diameter), 3D structures called organoids that closely mimic the brain environment. These organoids allow us to study diseases on an individual patient level. You can read about how Synapticure is using a similar technology to study Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD) here.
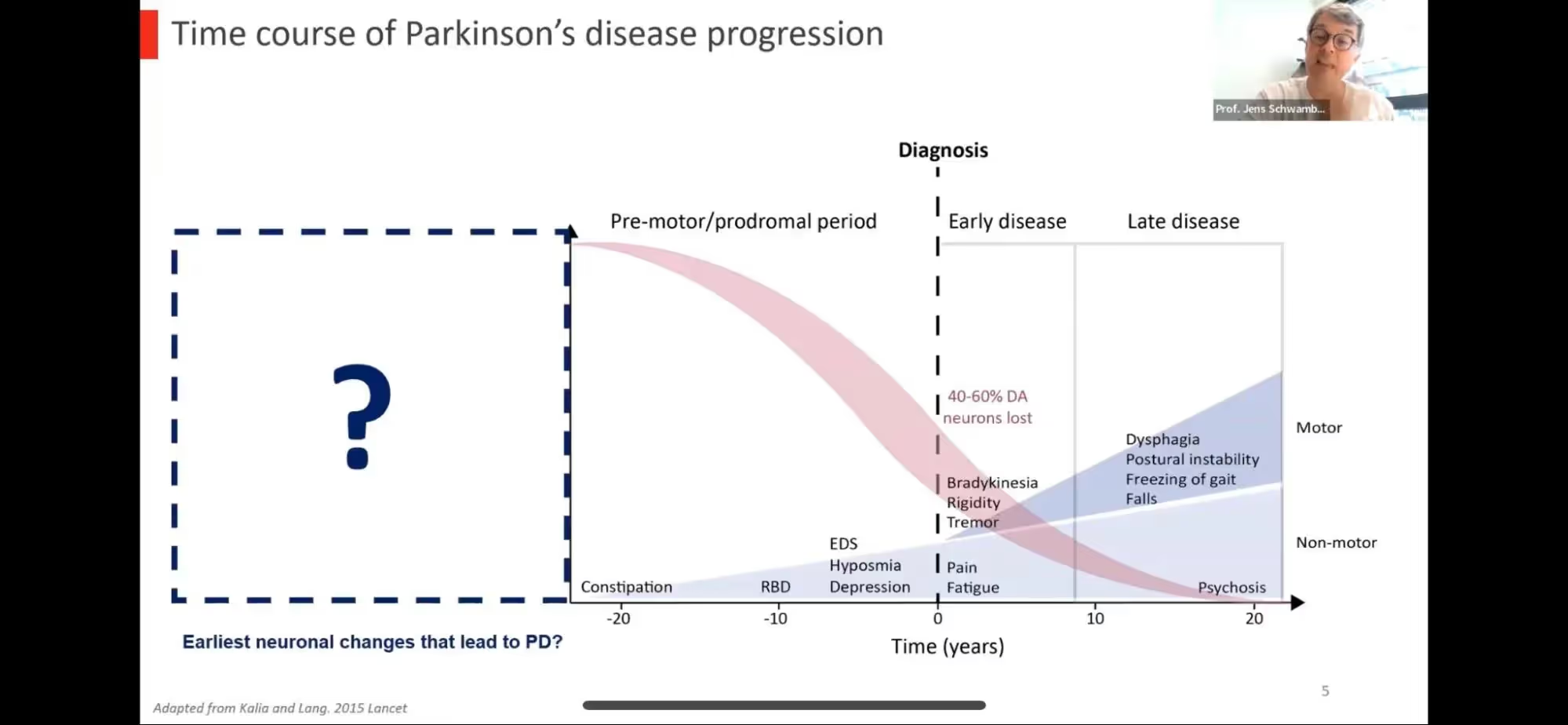
Dr. Schwamborn started with an introduction to PD including the general time course noting that symptoms actually occur after there’s already been a significant loss of dopaminergic neurons or brains cells (40-60%) which means there’s a significant prodromal or “pre-motor” phase when dopamine neurons are dying but there are no clear clinical symptoms. This phase is estimated to begin 10-20 years prior to clinical symptoms or possibly even earlier; maybe even as early as the developmental stages of an embryo where the predisposition to develop PD may first begin. In science, we often refer to the “two-hit” or “multiple hit” hypothesis where there’s not ONE event or exposure that causes a disease but rather, two more things may need to occur in order for disease to develop. For example, if you have a genetic predisposition for PD (e.g. a mutation in the LRRK2 gene), you have about a 50% chance of developing PD so there must be some secondary factor that determines who gets PD and who does not. This early developmental or pre-motor phase would be an ideal time to intervene to help prevent or delay the onset of PD.
Dr. Schwamborn goes on to discuss mutations in the GBA gene which is the most common risk factor for PD. Every individual carries two copies of any gene present in our genome. If one has a mutation in BOTH of their GBA genes, it causes Gaucher disease but if there is only a mutation in ONE of the genes, it increases the risk of PD. GBA encodes a protein called glucocerebrosidase which is involved in processes that aid in the breakdown of other proteins and clearance of defective or damaged parts of the cell. Mutations in GBA can result in improper functioning of glucocerebrosidase which, in turn, can ultimately lead to cell injury and cell death.
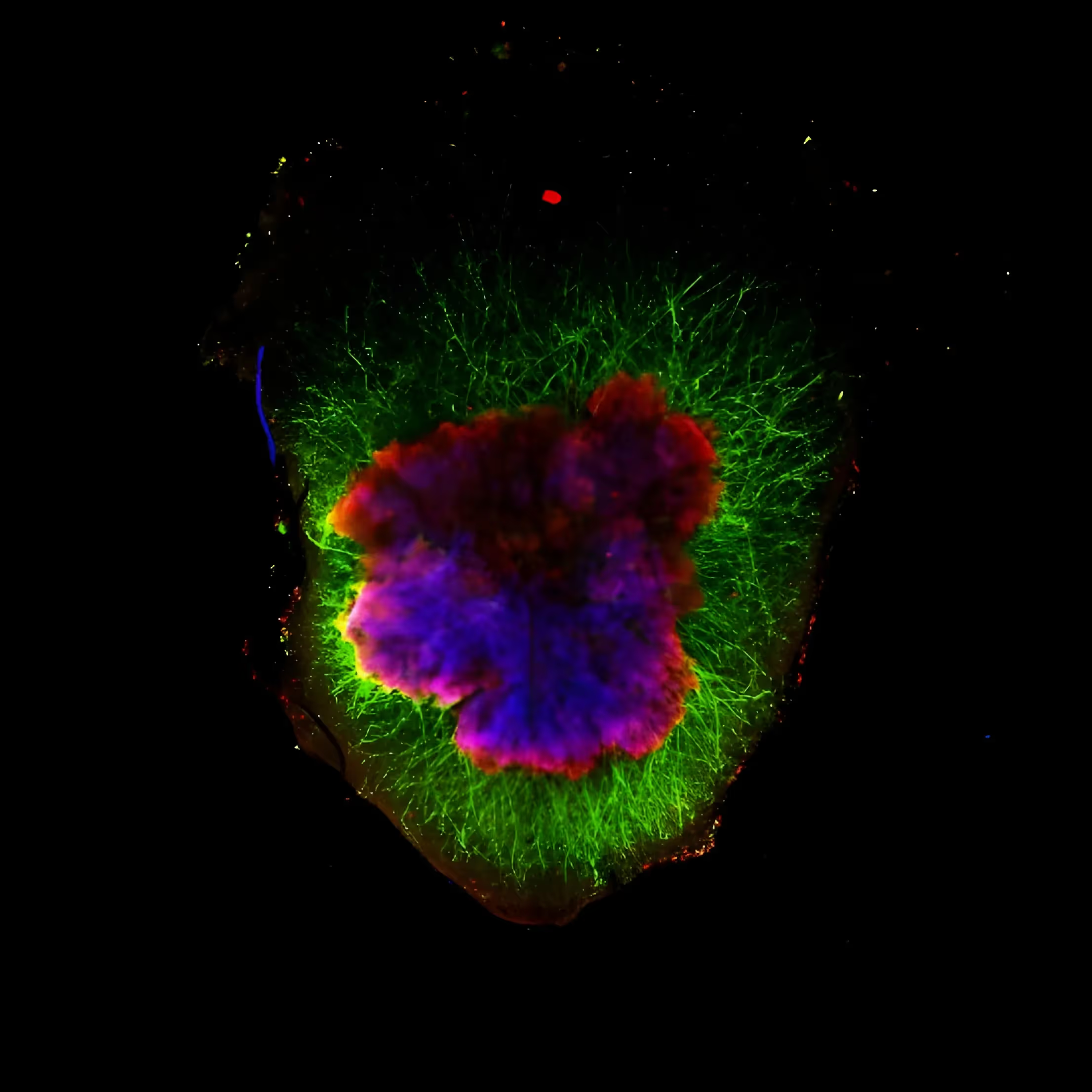
In his lab, they produce organoids that mimic the “midbrain”, the area in human brains where dopaminergic neurons are lost in PD. The cells in these organoids produce dopamine (they are “dopaminergic”) and show other features of mature human dopaminergic neurons NOT seen in rodent models of PD. They also have other support cells seen in human brains known as astrocytes. When these organoids are treated with 6-hydroxydopamine (6-OHDA; used to kill dopamine neurons as a model of PD in other laboratory settings), there is loss of dopaminergic neurons, as expected. Dr. Schwamborn’s team has developed various tools to detect neurodegeneration in the organoids (e.g. loss of cells and increased fragmentation of the remaining neurons).
Because 6-OHDA injury is an artificial model of PD, they then wanted to use organoids made from stem cells derived from people with GBA-associated PD. These PD organoids showed similar disease traits to the ones observed in patients including:
- Reduced activity of glucocerebrosidase and an overall decrease in the amount of glucocerebrosidase in later stages
- Distorted localization of the glucocerebrosidase
- Reduction in the amount of tyrosine hydroxylase (Surprisingly, the TH concentration PER neuron is actually HIGHER in PD-organoids, perhaps in an attempt to compensate by creating more dopamine but this overproduction can increase oxidative stress leading to more degeneration down the road)
- Reduced complexity, increased fragmentation, and lower electrical activity of the remaining neurons (signs of early degeneration)
Interestingly, mutations in other genes known to cause PD such as LRRK2 and 3xSNCA (alpha-synuclein triplication) result in changes that look the same when the organoids are 90-days old but the temporal pattern of changes to get there are very different. Organoids with LRRK2 mutations are normal for the first 30-60 days then decline. Those with 3xSNCA have normal or even more dopaminergic neurons in the beginning but then there is a very sharp decline around days 50-60. To further understand why these cells degenerate, they looked at gene expression and found that the networks of genes associated with differentiation (becoming more mature) are decreased while expression of genes associated with stem cells are increased. They also found evidence to suggest that persistence of stem cell markers would lead to premature aging in GBA-PD.
They did a second similar study but instead of GBA, looked at 3xSNCA and saw similar pathology as seen in PD including elevated levels of alpha-synuclein and phosphorylated synuclein that is visible in all stages of differentiation. This leads to more loss of dopaminergic neuronal loss at LATER stages than that in GBA-PD organoids as well as significant loss of dopamine production. This is the same in other organoids from patients with PD due to other gene mutations including LRRK2, PINK1, DNAJC6.
Perhaps most exciting, in the linked study PINK1-associated PD above, they tested a compound that allowed a rescue of the pathological changes seen. In a second study, with the DJ1 gene (yet another cause of PD), they developed compounds that addressed the molecular mechanisms affected in patients with these mutations resulting in full rescue of the dopaminergic neurons!
While all of the above studies have focused more on the “neurodevelopmental model” of PD, he also showed evidence that they can also mimic what happens in the aged brain. Examination of postmortem brain samples from patients with PD due to a mutation in the PARK2 gene (parkin) showed reduced number of astrocytes (support cells to help neurons get nutrition, remove excess neurotransmitters like dopamine, and regulate the blood brain barrier). Midbrain organoids created from stem cells from these patients showed the same pattern! They analyzed all the cell types in the organoids and they found that not only are there dopaminergic neurons, there are also those that produce other neurotransmitters such as glutamate, GABA and serotonin. But the brain is not only neurons and astrocytes. There are also immune cells, such as microglia, as well as blood cells that are involved in neuroinflammation and formation of vasculature and the blood-brain barrier. They have been able to create microglia from stem cells that act as microglia should. After much trial and error, they were able to develop a method to grow the midbrain organoids with neurons and astrocytes along with microglia. When put together, they “self-assemble” into structures called “assembloids” which are very similar to what we see in the human midbrain including similar gene expression. Inclusion of microglia more closely resembles or recapitulates the brain environment and significantly affects cell death, oxidative stress and neuronal functionality in the assembloid.
These assembloids can be created in large numbers allowing scientists to rapidly screen different therapeutic approaches prior to human studies. Only the most promising treatments will be taken to clinical trials hopefully resulting in less failures. Additionally, this platform can not only help finding a single effective therapeutic but also help understanding which patients may benefit from a combination of drugs. In fact, being able to derive models in the lab directly from patients (as opposed to those from genetic cell or mouse models) allows scientists to better represent patient-to-patient variability and better understand the heterogeneity of PD. Suffice it to say that this extremely exciting research is sure to result in rapidly expanding knowledge about how PD starts and progresses, and most importantly, how it might be stopped.
Header image courtesy of Jens Schwamborn, PhD










.png)


